An estimated 7.3% of the population is affected by Type II Diabetes Mellitus (T2DM), making it one of the most prevalent chronic diseases worldwide. This figure rises to as many as 34% of all adults in some countries. Approximately 1 in 3 adults are prediabetic, and many adults are undiagnosed. Historically, T2DM has been considered a disease that increases with age, but more and more children, teens, and young adults are now being diagnosed with both prediabetes and T2DM.
Prediabetes and T2DM are characterized by impaired glucose tolerance and decreased insulin sensitivity. This means that after eating, blood sugar levels increase to above what the body can safely tolerate before pancreatic β cells in the islets of Langerhans respond by secreting insulin. Additionally, in Type II diabetics, this insulin is not as effective at prompting glucose absorption from the blood stream as it is in non-diabetics. This is known as insulin resistance. This cycle of chronic elevated blood glucose leads to nerve damage in the eyes and peripheral limbs, kidney damage, increased risk of stroke and heart attack, and can be part of more widespread imbalanced hormone feedback loops with systemic effects.
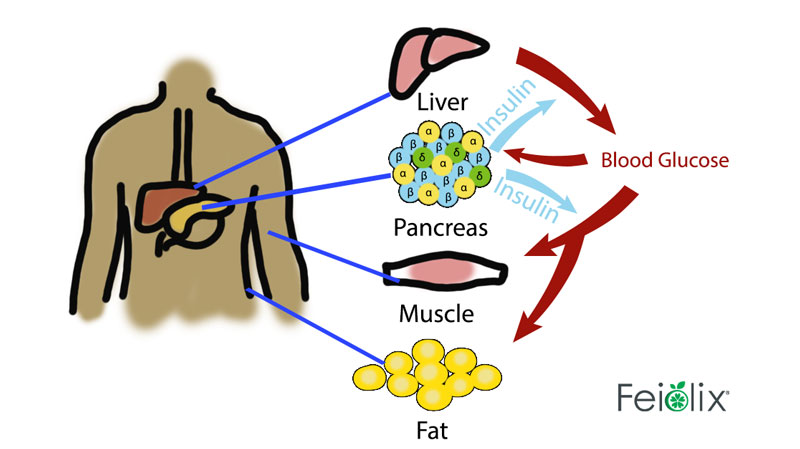
(adapted from https://bmcbiol.biomedcentral.com/ (Q&A: insulin secretion and type 2 diabetes: why do β-cells fail?))
Figure: sugar->blood glucose->detection by pancreatic islet of Langerhans β cells-> stimulation of insulin secretion-> absorption of glucose by skeletal muscle from blood
An oral glucose tolerance test and haemoglobin A1C (HbA1C) are used to diagnose prediabetes and T2DM. Blood sugar levels between 140 and 199, and/or an HbA1C level of 5.7% – 6.4% are considered prediabetic and patients are often advised to make diet and lifestyle changes to prevent further disease progression into T2DM. When blood sugar levels are above 200, and HbA1C levels are above 6.4% a patient is considered to have T2DM. Treatment often includes pharmaceuticals, such as metformin which increases insulin sensitivity, synthetic insulin administration, or pharmaceuticals that stimulate insulin release. While effective, these treatment regimens frequently cause side effects and negatively impact daily life.
Recommended dietary changes are intended to reduce the glycemic index of foods consumed by removing simple sugars and highly processed carbohydrates in favor of complex carbohydrates. The more complex a carbohydrate is, the longer it takes to be broken down by amylase enzymes in the body, leading to a lower and slower post-prandial (after eating) blood-glucose peak, and the lower glycemic index it is considered to have. While these dietary changes are crucial to prevent and reverse the progression of T2DM, there are additional dietary modifications that can actively target and support blood glucose homeostasis.
Top 10 swaps from high glycemic to lower glycemic index foods
| High GI foods | Moderate and Low GI alternatives | ||
| Rice milk | 86 ± 7 | Soy milk | 34 ± 4 |
| Cornflakes | 81 ± 6 | Rolled oats | 55 ± 2 |
| White potato mash | 78 ± 4 | Sweet potato mash | 63 ± 6 |
| White wheat bread | 75 ± 2 | Rye pumpernickel bread | 43 ± 2 |
| White rice | 73 ± 4 | Barley | 28 ± 2 |
| Boiled taro | 53 ± 2 | Boiled carrots | 39 ± 4 |
| Banana | 51 ± 3 | Apple | 36 ± 2 |
| Watermelon | 76 ± 4 | Orange | 43 ± 3 |
| Ice cream | 51 ± 3 | Yogurt (Greek) | 11 ± 2 |
| Table sugar | 65 ± 0 | Stevia | 0 |
These recommendations are based on the glucose-stimulated insulin-secretion pathway, in which glucose in the blood is detected and absorbed by β-cells of the islets of Langerhans, which synthesize and release insulin. Simple glucose-stimulated insulin-secretion is a primary pathway and is well understood, but fatty acids, amino acids, hormones, and various bioactives and pharmaceuticals also influence insulin release.
While modulating blood glucose levels by reducing the amount and speed at which glucose enters the bloodstream is an important and effective modification, this pathway can also be used to stimulate insulin secretion at lower levels of blood glucose. Incretin hormones are intestinal hormones which enhance the secretion of insulin. The most active and understood of these incretins are glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide.
Pharmaceutical GLP-1 receptor agonists (mimicking GLP-1) and GLP-1 degradation prohibitors (prolonging the life of GLP-1) have been used since 2005 to treat T2DM by stimulating insulin release at lower blood glucose levels. GLP-1 receptor agonists only support gene transcription of insulin in the presence of blood glucose, so there is no risk of hypoglycemia.
However, there are also natural dietary components that act as a GLP-1 receptor agonists. The most promising and well understood compound that behaves in this way is abscisic acid (ABA). Another class of food derived compounds that stimulate insulin secretion are urolithins– microbially transformed antioxidants, ellagitannins.
ABA is well known as a plant stress hormone involved in fruit ripening and the separation of fruit from stem. The highest concentrations of ABA have been documented in New Zealand feijoa fruits, followed by avocados, citrus, soybeans, figs, bilberries, maize, apricots, apples, and tomatoes.
ABA is also produced and released by human pancreatic β-cells, granulocytes and monocytes at very low levels, meaning that this chemical pathway exists in both plants and animals. We humans have receptors (LANCL2) that respond to ABA.
Top 10 food sources of ABA
| Foodstuff | ABA level (mg/kg) |
| New Zealand Feijoas | 4.29 |
| Avocados | 2.0 |
| Citrus | 1.25 |
| Soybean | 0.79 |
| Fig | 0.72 |
| Bilberry | 0.4 |
| Maize | 0.33 |
| Apricot | 0.32 |
| Apple | 0.30 |
| Tomato | 0.20 |
Human LANCL2 (lanthionine synthetase C-like 2 proteins) receptors are expressed across innate immune cells, stem cells, β-cells in the pancreas, and muscle cells. In the β-cells in the pancreas (the insulin-secreting cells), ABA binding to the LANCL2 receptors upregulates glucose transporters in skeletal muscle, thereby decreasing blood glucose and increasing muscle glycogen stores for immediate utilization as energy. In addition, ABA binding to the LANCL2 receptors starts a molecular cascade and positive feedback loop that enhances and extends the glucose–induced secretion of insulin.
Similarly, consuming foods high in a subclass of ellagitannins called catechins (green tea, apples, and feijoas) leads to the production of urolithins by the gut microbiota. Urolithins bind to an L-type calcium channel on the B cells of the pancreas, leading to an influx of calcium into the cell. This depolarization triggers the secretion of insulin when lower levels of glucose are present.
This means that the consumption of foods high in ABA and ellagitannins supports insulin secretion. Support of insulin secretion is crucial for T2DM and prediabetes patients on reduced glycemic diets because these individuals are insulin-insensitive but now must respond to lower peaks of blood glucose which are triggering a lower insulin response than what they are accustomed to.
By supporting insulin secretion with ABA and ellagitannins, their response can remain within the normal range. This is why a combination of reduced blood glucose and improved insulin secretion together support better blood glucose management.